Abstract

Background SB12 is a humanized monoclonal antibody that has developed as a proposed biosimilar to reference eculizumab (ECU) and its pharmacokinetics (PK) similarity compared with ECU in healthy subjects has demonstrated. Also, SB12 Phase III study has demonstrated equivalent clinical efficacy by evaluating lactate dehydrogenase (LDH) and comparable safety, PK, pharmacodynamics (PD), and immunogenicity between SB12 and ECU in paroxysmal nocturnal hemoglobinuria (PNH) patients (Jun Ho Jang et al., EHA 2022).
Objective To explore the robustness of the primary efficacy results of SB12 Phase III clinical study (NCT04058158) by conducting sensitivity analysis.
Methods This was a Phase III randomized, double-blind, multi-national, cross-over study in patients with PNH. A total of 50 patients who aged ≥ 18 years with a confirmed diagnosis of PNH and ≥ 1.5 upper limit of normal range (ULN) of LDH without previous exposure to a complement inhibitor were included. Patients were randomized in 1:1 ratio to either treatment sequences and received either SB12 or ECU by IV infusion every 7 days for the first 4 weeks (initial phase) and 900 mg for the fifth week, followed by 900 mg every 14 days (maintenance phase). The treatment was switched to ECU or SB12 at Week 26, and switched treatment was provided until Week 50.
The primary endpoints of this study were LDH level at Week 26 (equivalence declared if the two-sided 95% confidence interval (CI) of the least squares means (LSMeans) difference in LDH level at Week 26 between SB12 and ECU lied within the pre-defined equivalence margin of [−1.2 × ULN, 1.2 × ULN] = [−337.2, 337.2], where ULN = 281 U/L) and time-adjusted area of under the effect curve (AUEC) of LDH from Week 14 to Week 26 and Week 40 to Week 52 (equivalence declared if the two-sided 90% CI of the ratio of geometric LSMeans in time-adjusted AUEC of LDH between SB12 and ECU lied within the pre-defined equivalence margin of [0.77, 1.29]).
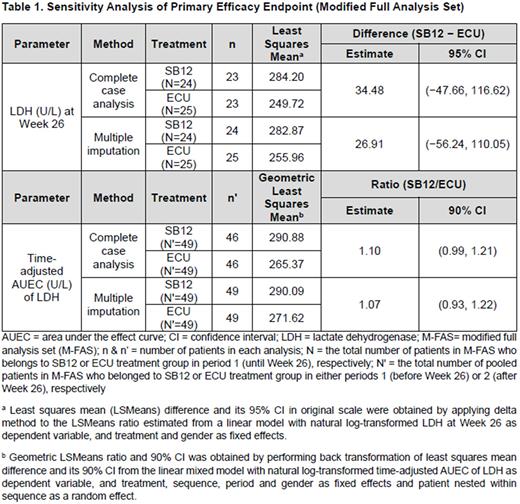
Sensitivity analysis was performed using complete case analysis or multiple imputation method in modified full analysis set (M-FAS). Complete case analysis is based on complete LDH data, without imputation on missing LDH data. With regard to multiple imputation that assumes missing at random (MAR), missing LDH data was imputed for patients who dropped out of the study prior to the primary analysis timepoint. Missing LDH data was imputed by fully conditional specification (FCS), with the non-missing LDH data at previous visits, treatment sequence, and gender as covariates. In case of sensitivity analysis of LDH level at Week 26, results of linear model based on loge-transformed LDH values were combined first using Rubin's rule and then delta method was used later to report mean difference and 95% CI in original scale.
Results In the sensitivity analysis of LDH level at Week 26, for the complete case analysis in M-FAS, estimated LSMeans difference (SB12 − ECU) of LDH level at Week 26 between SB12 and ECU was 34.48 (95% CI [−47.66, 116.62]). In the multiple imputation analysis, estimated LSMeans difference (SB12 − ECU) of LDH level at Week 26 between SB12 and ECU was 26.91 (95% CI [−56.24, 110.05]). Sensitivity analysis results of LDH level at Week 26 provided consistent result with primary analysis (SB12 − ECU: estimated LSMeans difference 34.48, 95% CI [−47.66, 116.62]).
Meanwhile, sensitivity analysis of time-adjusted AUEC of LDH from Week 14 to Week 26 and from Week 40 to Week 52, estimated ratio of geometric LSMeans in time-adjusted AUEC of LDH (SB12/ECU) between SB12 and ECU was 1.10 (90% CI [0.99, 1.21]) for the complete case analysis in M-FAS. In the multiple imputation analysis that assumed missing data were MAR, estimated ratio of geometric LSMeans in time-adjusted AUEC of LDH (SB12/ECU) between SB12 and ECU was 1.07 (90% CI [0.93, 1.22]). Sensitivity analysis results of time-adjusted AUEC of LDH from Week 14 to Week 26 and from Week 40 to Week 52 showed consistent result with primary analysis (SB12/ECU: estimated ratio of geometric LSMeans 1.08, 90% CI [0.95, 1.23]).
Conclusion This sensitivity analysis results showed consistent results and validated robustness of the primary analysis and it supports the therapeutic equivalence between SB12 and ECU.
Disclosures
Demichelis:Abbvie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; ASH: Research Funding; Teva: Consultancy; Gilead: Consultancy. Kim:Samsung Bioepis: Current Employment. Park:Samsung Bioepis: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal